UTI Vaccine
Recurrent UTIs can be effectively treated with a simple UTI vaccine where other methods of mainstream management are no longer working.
Uromune®
Patients often rely on repeated and prolonged courses of antibiotics. This is particularly true in prostatitis where current international guidelines recommend 2-4 weeks of antibiotic treatment.
However, with the alarming rise of antibiotic resistance, described as one of the biggest risks to global health in our lifetime, and with the World Health Organisation triggering a Global Action Plan to combat antibiotic resistance, there is a pressing need to find viable alternatives.
Recently the use of vaccine / immunostimulation in patients with recurrent infections has become more popular. The science behind this won a Nobel Prize for medicine in 2010.
Uromune is a new and exciting vaccine for treating recurrent urinary tract infections (UTIs).
This UTI vaccine is composed of the inactivated whole bacteria of the four most common bugs that cause UTIs in men and women (Escherichia coli, Klebsiella pneumoniae, Proteus vulgaris and Enterococcus Faecalis).
Rather than being injected, it is administered daily over three months via a pineapple flavoured spray. A few sprays under the tongue each day is all that is required.
Once administered it interacts with the immune system, resulting in long term protection from UTIs in the patient.
If you are interested in Uromune, we are very happy to discuss any questions you may have. Please call us on 0118 920 7040 or send us an email to info@theforburyclinic.co.uk.
At the forefront of Uromune trials in the UK
We are proud to have presented and published nationally and internationally on the first experience in the United Kingdom of Uromune in preliminary cohorts of both men and women with recurrent UTIs that have failed mainstream managements including antibiotic prophylaxis.
At Reading we conducted the first randomised controlled trial in the use of vaccines for urinary tract infections. The vaccine was used in several patients on a named-patient basis and the interim results of our study published in the British Journal of Urology.
Initial research data suggested a 70-80% reduction in infections in a one-year period for patients who have tried all other treatments, which looks very promising.
Our conclusions from these preliminary results, suggest the Uromune vaccine is both effective and safe in UK men and women with recurrent UTIs, and may offer a potentially viable alternative to antibiotics.
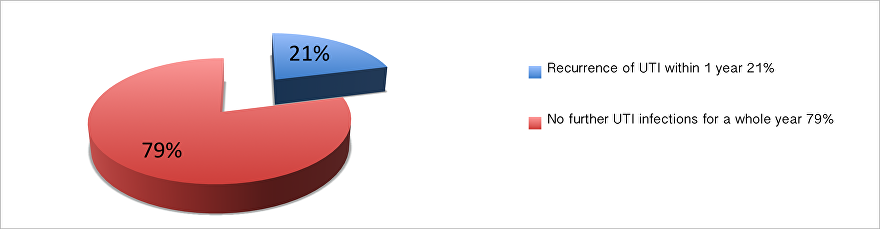
More recent research further supports the clinical efficacy and safety in reducing the incidence and preventing recurrence of UTIs following Uromune vaccination.
Sublingual vaccination with Uromune (MV140) prevents recurrent urinary tract infections in women. Preliminary results from a multicentre, Spanish-UK randomized, double-blind, placebo-controlled phase III trial
Presenter: Bob Yang
Introduction: Antibiotic prophylaxis for recurrent urinary tract infections (rUTI) in women and the rise in antibiotic resistance has resulted in tremendous costs, treatment failure and patient morbidity. MV140 (Uromune) is a polybacterial sublingual vaccine consisting of whole-cell inactivated bacteria (E. coli, K. pneumoniae, E. faecalis and P. vulgaris) that has shown clinical benefit in previous observational studies. Results from a randomized placebo-controlled trial (RCT) were awaited to confirm its clinical efficacy.
Patients and Methods: A phase III multicentre, double-blind, parallel group RCT, enrolled 240 women aged 18-75 with rUTI from Spain and UK. They were randomly allocated to receive placebo for 6 months or MV140 (active) for 3 or 6 months, in a 1:1:1 ratio. Primary and major secondary endpoints were the number of UTIs and UTI-free rate in the 9-month efficacy period, respectively.
Results: The median number of UTI episodes was 3.0 [interquartile range, IQR, 0.5-6.0] for placebo group compared to 0.0 [IQR, 0.0-1.0] in both groups receiving MV140 (P≤0.001). A significant increase (over 2-fold) in the UTI-free rate was observed in the treatment groups (P≤0.001) compared to the placebo group (25.0%). Only 5 subjects reported drug related adverse reactions, all were non serious, 2 in the placebo group and 3 in the MV140 3-month group.
Conclusions: The preliminary late-breaking analysis of this first MV140 RCT shows clinical efficacy and safety in reducing the incidence and preventing recurrence of UTIs. Clinical utilisation of this novel sublingual bacterial vaccine may offer women an effective evidence-based alternative to antibiotic prophylaxis in the management of rUTI.
More information on Uromune®.
Fighting a wee problem…with a simple spray! Doctors discover breakthrough vaccine which could spell the end to painful UTIs. Article on UTI Vaccine and interview with Mr Steve Foley, published Mail on Sunday 30 December 2017
Groundbreaking’ UTI vaccine could stop infections for nine years. The Independent on Sunday (7 April 2024)
Vaccine that can stop UTIs for NINE years – in drug that could prove a saviour for up to 1.7 million Britons. The Mail on Sunday (7 April 2024)
‘Game changer’ UTI vaccine stops infection for nine years The Sunday Telegraph (7 April 2024)
Vaccine prevents UTIs for up to 9 years in more than half of people Cosmos Magazine published Sunday 7 April 2024
Next Steps
Our UTI Vaccine Specialists
Get fast access to leading specialists for the swift diagnosis and treatment of urological conditions in a private clinic environment.
If you would like more information or wish to arrange a consultation with one of our specialist consultant urological surgeons then please either Call 0118 920 7040 or complete the form below.
Insured patients
Contact your GP and ask for a referral to the Urology Partnership.
All consultations, investigations and treatments are covered by major insurance companies (depending on policy).
Funding your own treatment
Self-funding initial consultation fee is £205. Follow up fees are £165.
Consultation charges are exclusive of any tests and other investigations that the consultant may wish to carry out.




